Physics
Lubricant Properties as a Function of Temperature
The properties of lubricants, similar to water, change as a function of temperature and pressure. Unlike water, lubricants are, with few exceptions, mixtures instead of pure or nearly pure substances. Note that the definition of pure in this context is not defining pure as a lack of contamination. If an uncontaminated pure substance is willfully mixed with different uncontaminated pure substance, the mixture is no longer a pure substance, but it is not contaminated because the mixture was desired. Because the different pure substances that compose a mixture have different melting and boiling characteristics, the melting and boiling points for lubricants are not well defined.
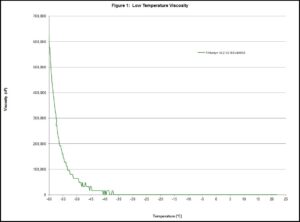
Instead of defining the melting point, lubricants are described by determining the pour point . The description has room for interpretation and ASTM D97 does not have good repeatability. There are other methods to capture this characteristic, like using a rheometer to measure the viscosity as the temperature decreases. This method requires the pour point to be an apparent viscosity that has been declared or agreed upon prior to the test being run. Below is a graph of data collected on Tribosyn® 612-32. This oil is approximately 32 cP at 40°C. The viscosity at ambient is so low that the device that was used to collect the data did not detect enough resistance to report a nonzero value until the oil was below -35°C.
At ambient temperatures, the pure component vapor pressure of the constituents most oils and greases is low enough that the lubricant loses negligible mass over time. As temperature increases, more volatile components enter the vapor phase as the vapor pressure of the components increase and the lubricant begins to lose mass at a measurable rate. Using the Antoine equation, it is possible to estimate the vapor pressure of a substance at low temperatures by determining the constants using data at higher temperatures. If enough vapor escapes the lubricant as temperature rises, and the lubricant is capable of burning, then it is possible for the mixture of air and vapor to ignite. The flash point of a substance is the temperature where this occurs. The fire point is the temperature where the fire stays lit.
The viscosity of a lubricant decreases as temperature increases. The viscosity index (VI) of a lubricant is a number derived from the behavior of a standard naphthenic at zero to 100 for standard parafinnic oil. The higher the VI, the less the oil changes as temperature changes. Many synthetic oils have VI that fall outside of the zero to 100 range. The viscosity of oils can be predicted with two data points as long as the data and predictions are not close to the pour point. Because some of the lubricant is lost at high temperatures, the composition changes over time. That will affect the viscosity even if no other reactions occur like oxidation and/or thermal decomposition.
The operating range of a lubricant can be generalized across many applications, but is best expressed for a specific application. The common failure mode for a lubricant at low temperature is the lubricant becomes so immobile that the application can no longer function. Some applications are much more tolerant of immobility than others. For example, the bearings in locomotive trucks can tolerate a much more viscous lubricant than the bearing in a computer cooling fan. Volatility, oxidation, and thermal decomposition commonly determine the upper operating range of a lubricant. A general upper operating range can be given using a lube for life application as a basis. Applications that allow for re-lubrication can exceed that temperature depending on the residence time of the lubricant and how long the lubricant can survive at that temperature.
Click here to learn more about Physics topics.